Nacional cocoa from Ecuador is the source of the fine flavour cocoa called Arriba. The exact origin of the Nacional type is not known, but it is generally considered as native to Ecuador. Recent studies using RFLP probes indicated that Nacional cocoa genotypes are genetically different from the Forastero, Criollo or Trinitario groups. Data are presented here, and briefly discussed, on the level of heterozygosity and genetic diversity of 416 genotypes characterised, including more than 100 Ecuadorian genotypes. The Ecuadorian types constituted two populations: one group having a low level of heterozygosity (average of 5%) and row genetic diversity, representing part of the native Nacional group, and a second group with a higher level of heterozygosity (average of 32%) that resulted from introgression with cocoa types from Venezuela and Trinidad. Due to their higher productivity, hybrids now predominate over Nacional types.Resistance to witches’ broom disease, yield improvement and consistent Arriba flavour were defined as selection priorities in Ecuador. These criteria were studied through a sub-sample of clones collected in various locations, representing the Ecuadorian genetic diversity (EB clones). No immunity to witches’ broom disease was detected but a relationship between vigour and disease incidence was found suggesting that more brooms develop on more vigorous trees. The sensory evaluation indicated that cocoa trees which give rise to beans with significant Arriba flavour are not distributed according to their geographic origin or their genetic status. In fact, Arriba flavour has been found in both Nacional and hybrid types. The relative importance of the genetic and environmental factors on the Arriba flavour are yet to be determined.
Introduction
The well-known Ecuadorian cocoas, derived from the Nacional variety, are considered to be fine-flavoured. The production of this specific Arriba flavour not only depends on genetic factors, but also on the post-harvest fermentation and the processing in the factory.
In 1915, Ecuadorian cocoa production reached 50,000 tonnes when Ecuador was the second largest producer after Ghana. But the arrival of witches’ broom disease in the 1920s led to a strong decline in cocoa cultivation. From the beginning of the 1930s, the quality of Ecuadorian cocoa has deteriorated due to the introduction of Trinidad and Venezuelan clones collected for resistance to witches’ broom disease. An increasing number of the trees planted today are hybrids from these clones (Lerceteau et at 1997). This was reflected in ICCO’s decision to consider Ecuador as a country that produced only 75% fine flavoured cocoa. Economically, this was a blow to the exporters as the premiums on cocoa prices decreased, and the industry is also suffering as good quality becomes harder to find.
In Ecuador, cocoa is primarily grown in the western coastal regions between sea level and 400 m. There are three main zones: The northern zone includes Esmeraldas, Santo Domingo and Chone areas. The plantations mainly contain hybrids (Nacional x Trinitario). Yields are relatively low with a favourable climate for pathogen devecopment The central area is in the Guayas river valley in Los Rios province. The cocoa trees are old and severely affected by the witches’ broom disease but the soil
and climatic conditions are suitable for cocoa cultivation. In the southern area, the province of El Oro is characterised by a dry season lasting up to eight months. The low humidity of the zone makes it less favourable to the development of cocoa diseases. Cocoa has been planted as a substitute for banana in this area over the past fifteen years (Petithuguenin and Roche 1995).
The witches’ broom pathogen, Crinipellis perniciosa (basiodiomycete), is endemic to wild cocoa and it is spreading in a natural way to cultivated cocoa in all countries around the Amazon basin. The fungus attacks young meristematic tissues causing hypertrophic and prolific growth on vegetative shoots and flower cushions (brooms), but infects pods (Van der Vossen 1997, for review). Different strains of witches’ broom disease were detected, and four major biotypes were identified, The most virulent biotype is found in Columbia and Ecuador and the least virulent comes from Trinidad (Zadoksl997, for review).
Host resistance is the only long-term answer to this devastating pathogen, since disease control measures, such as intensive sanitary pruning and fungicide spray, are only partly effective and generally not economic. The search for resistance started in the 1930s, and was followed up in several countries including Trinidad, Brazil and French Guiana. In fact, cocoa germplasm evaluation in the field showed a large variation in the disease symptoms resulting in detection of a few resistant clones like Scavina genotypes, especially SCA 6.
In view of maintaining a consistent production of Arriba fine-flavoured cocoa in Ecuador, Nestlé and several partners, including INIAP, have selected Nacional cocoa genotypes, through field evaluation, that are supposed to be tolerant to witches’ broom disease. This study summarises the results of the molecular and field data analysis performed for this project.
Material and methods
Plant material
DNA extraction and RFLP markers
Field data
Sensory evaluation
Samples of 1 50g of cocoa beans, fermented for two days, were roasted at 120°C for 20 minutes. The roasted beans were hulled and ground to give the cocoa liquor. A trained tasting panel (4 to 6 persons) evaluated the cocoa liquor samples, which were maintained at 45°C in a water bath during the sensory sessions.
Statistical analysis
Results
Genetic structure of populations studied
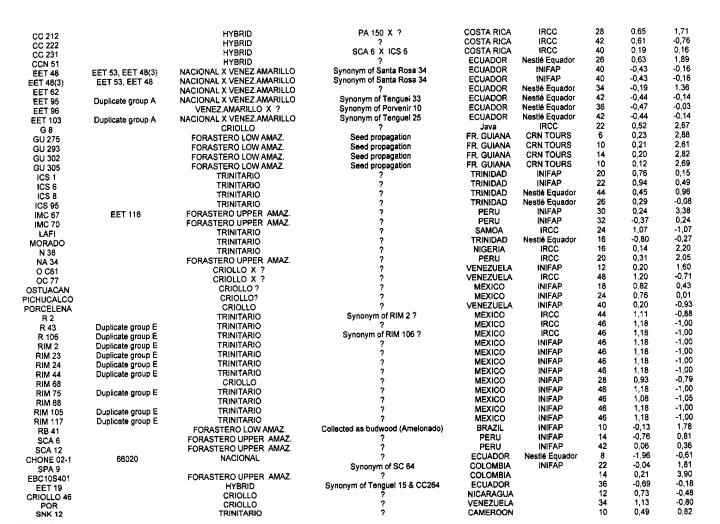
Genotypes from Peru. Only 28 Peruvian genotypes were analysed but all the 50 RFLP probes used were polymorphic with a total of 87 alleles. The average number of alleles per locus is 1.74. Despite the low number of genotypes studied it appears that these have specific alleles, which characterise the specificity of the genetic diversity of this area. Moreover all the RFLP probes tested were polymorphic, suggesting that Peru could be a centre of diversity for Theobroma cacao. The hierarchical clustering analysis (not shown here) tends to group the genotypes into three main clusters.
Cluster A is constituted of Scavina genotypes (SCA 6 and SCA 12), EET 109 and TAP 1 The level of heterozygosity is rather low (16.3% ~-/- 4.3%). The Scavina clones were collected by Pound in 1938 near Rio Ucayali according to the International Cocoa Germplasm Database of 1997.
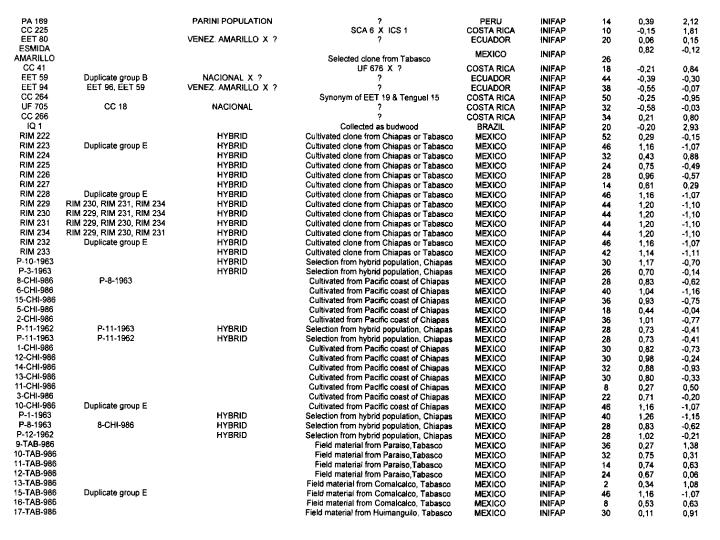
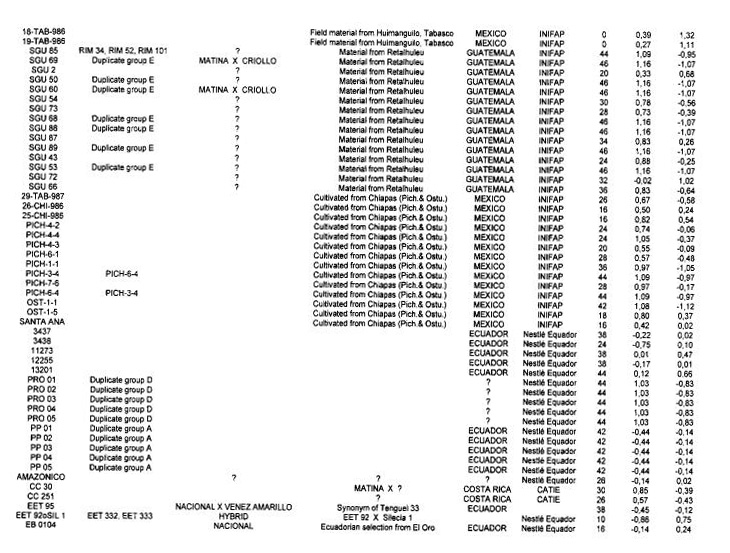
Genotypes from Mexico and Guatemala. The analysis of the 115 Mexican and Guatemalan genotypes with the 50 RFLP probes shows that 46 probes were polymorphic with a total of 77 alleles detected. The average number of alleles per locus is 1.67 indicating a low genetic variability. This genetic characteristic is also indicated by the high number of duplicates detected in this group (Annex 1).
The hierarchical clustering analysis (results not shown here) illustrates the high number of duplicates detected by the RFLP study and resulted in the detection of four clusters. Cluster A is represented by a single genotype (9-TAB-986), which comes from cultivated material in the Tabasco area, and cluster B encloses three genotypes (SGU 72, RIM 222 and RIM 224). Cluster C contains twenty-five genotypes with a heterozygosity level of 17.7 % *1- 9.7%. This group includes all the genotypes with a low level of heterozygosity, such as 18-TAB-986 (0%), 19-TAB-986 (0%), 13-TAB-986 (2%), 11-CHI-986 (8%), 16-TAB-986 (8%) and RIM 221 (10%), which are cultivated cocoa types except RIM 221 which comes from wild material.
Cluster 0 includes 86 genotypes with 51 duplicates showing a low genetic diversity.
The average heterozygosity level in this cluster is 40.3% (+1-8,1%), reflecting the likely hybrid status of these genotypes. Twelve out of the fifteen SGU types from Guatemala were distributed in cluster D suggesting a close relationship between Mexican and Guatemalan genotypes.
Genotypes from Costa Rica. The RFLP analysis performed on the 73 Costa Rican genotypes with the 50 RFLP probes shows that 44 probes were polymorphic with a total of 85 alleles detected. The average number of alleles per locus is 1.93. The hierarchical clustering analysis (not shown here) detected five clusters. Cluster A was composed of only two genotypes (CC 49 and CC 246). Twenty-four genotypes with an average heterozygosity level of 32.2% (+1- 8.5%) constitute cluster B. This group includes three UF genotypes (UF 676, UF 168 and UF 11) that cannot be differentiated. CC 225 is the only individual in cluster C; it is derived from the cross between Scavina 6 and ICS I from Trinidad. This genotype was obtained from the INIFAP collection and it is different from the genotype of the same name received from the CATIE collection, that belongs to group D. This result underlines the possibility of the mislabelling of this clone at INIFAP or at CA11E. Cluster D, with 17 genotypes, has a heterozygosity level of 35.2% (+1- 5.4%), similar to cluster A. It includes a majority of genotypes originating from crosses between Costa Rican genotypes and introduced genotypes, which would explain the high level of heterozygosity found.
Cluster E shows a lower heterozygosity level (23.8% +I- 11.3%) with individual values ranking from 0% (CC 33) to 50% (CC 264). This high variability in the levels of heterozygosity suggests that this group is heterogeneous.
Agronomic and flavour studies on Ecuador cocoa types
Witches’ broom resistance study. The significant coefficient of determination between trunk diameter and broom number (R20.53; p=O.0000B4) demonstrates a direct link between the vigour of the genotype and the number of brooms produced (Table 1). The larger the trunk diameter, the higher the number of brooms. This physiological relationship indicates that the disease impact appears to be more important on vigorous trees, such as hybrid forms than on homozygous trees from the pure Nacional group.
A better estimate of the resistance to witches’ broom disease in relation to tree vigour could be given by the ratio of the number of brooms to the total number of vegetative growing points of each genotype.
Discussion
The genetic characterisation of the Ecuadorian cocoas using molecular markers and phenotypic data (Lerceteau et at 1997) has allowed the detection of the specific diversity of Nacional genotypes. It is shown here that RFLP markers could also be used to differentiate the Ecuadorian genotypes according to their heterozygosity level. The self-compatible nature of Nacional cocoa (Enriquez 1993) may be one of the reasons for the low heterozygosity of this population. The diversity detected in the Ecuadorian accessions is due to the genetic origin of pure Nacional trees, on one hand, and the introduction of clones from Venezuela and Trinidad on the other hand.
Arriba flavour is assumed to have a specific origin in the genetic background Nacional, but due to the recent and multiple introgressions, the future production of Arriba flavour cocoa in Ecuador remains uncertain. The first sensory evaluations made on a representative sub-sample of several Ecuadorian genotypes clearly indicated that Arriba flavour could be found in the pure Nacional type and also in different hybrid cocoas growing in Ecuador. The relative importance of other parameters on the origin of Arriba flavour such as the growing area, bean fermentation at the farm level and the roasting in the factory remains unknown and has to be studied further.
Prospects for developing cocoa cultivars with an adequate level of resistance to witches’ broom disease are underway in several countries (e.g. Brazil - Pires et at 1999 and Luz et a!. 1999; Ecuador- Aragundi et a!. 1987; United Kingdom — wheeler 1999; French Guiana - Lachenaud and Ducamp 1996). However, due to the lack of a reproducible screening test, only field evaluations can be performed. In addition, field screening showed a very large variation in disease symptoms (brooms per tree), with no genotypes exhibiting complete resistance to witches’ broom infection and a resistance that is generally of a quantitative and incomplete nature. Only a few genotypes, such as SCA 6, gave limited evidence of resistance, but it seems that this genotype is inadequate under Ecuadorian conditions, certainly due to the strong aggressiveness of the pathogen strain. Other genotypes such as EET 233 have been reported to have resistance in Ecuador (Aragundi et al. 1987).
Our results suggest that tree vigour could also be, at least partly, a component of the disease impact For the time being, the multiplicity of the genetic, physiological and host-pathogen interaction factors implicated in witches’ broom disease suggest that clonal selection is the best method to achieve short-term progress. However, the clones selected must be acceptable agronomically and quality-wise (possessing Arriba flavour).
References
Aragundi J., C. Suarez and G. Solozano. 1987. Evidence of resistance of cocoa clone EET 233 to Moniliasis and witches broom. Pages 479-483 in Proceedings of the 10th International Cocoa Research Conference, Santo Domingo, Dominican Republic, May 17-23, 1987:


Biotech Glossary |
Bioinformatics |
Lab Protocol |
Notes |
Malaysia University |
Genetic Structure, Characterisation and Selection of Nacional Cocoa Compared to Other Genetic Groups
Dominique Crouzillat, Laurence Bellanger, Michel Rigoreau, Peter Bucheli and Vincent Pétiard
Centre de Recherche Nestlé, 101, Avenue Gustave Eiffe!, Notre-Dame D’Oé, B.P. 9716, 37097 Tours Cedex 2, France
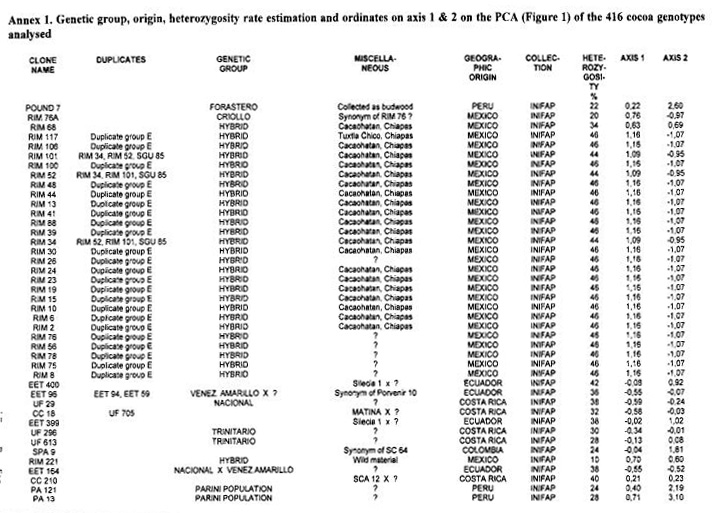
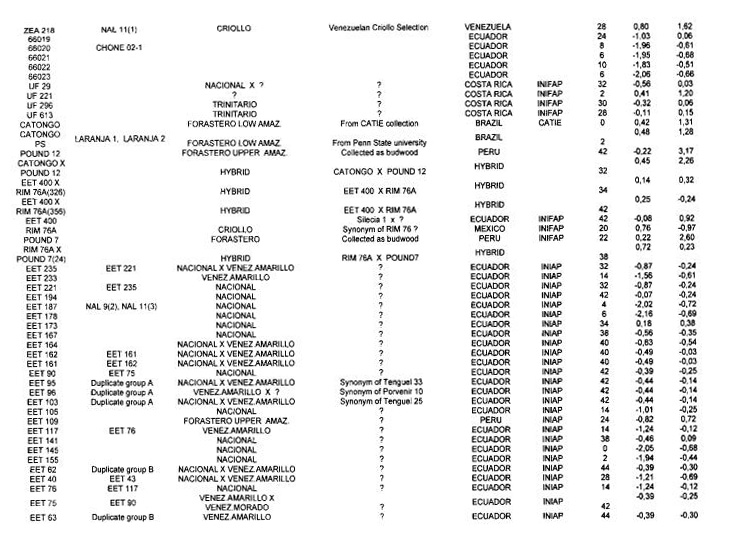
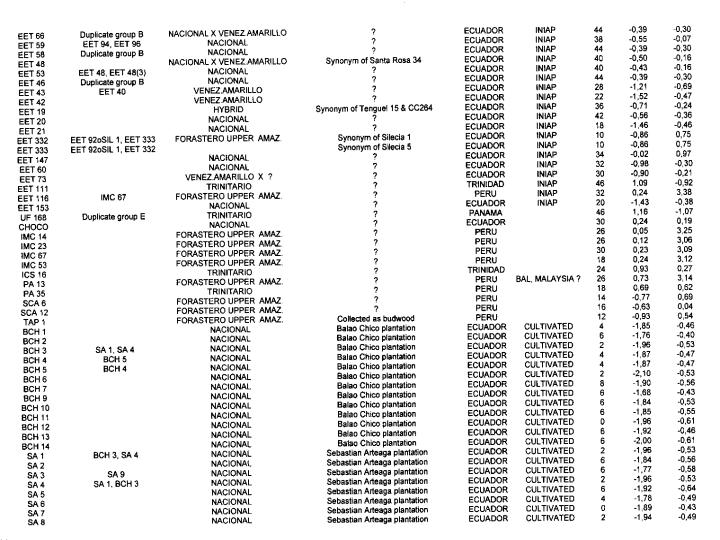
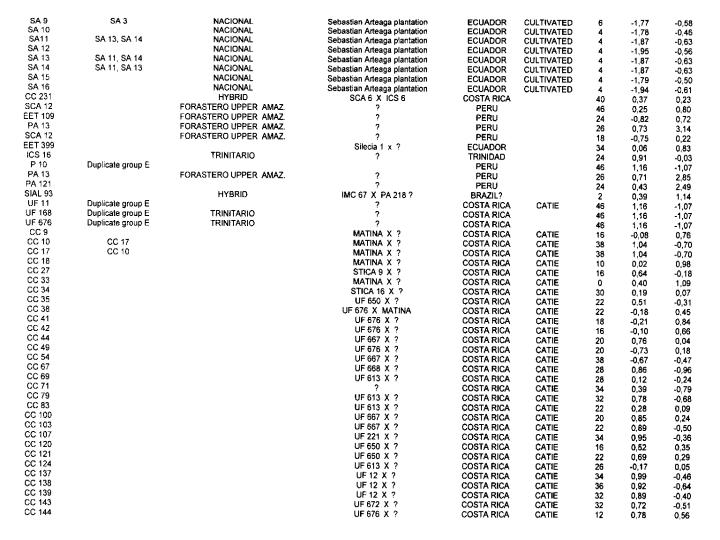
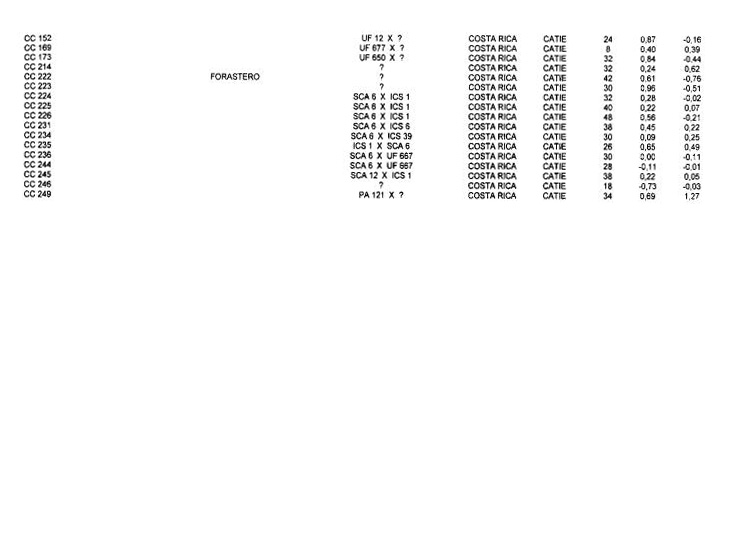
A total of 416 cocoa clones (Annex 1) from various collections were analysed including
more than 100 genotypes from Ecuador from INIAP, the Nestlé farm at Quevedo and
from two field locations at Sebastian Arteaga (SA) and Balao Chico (BCH) which are
80 to 100 years old plantations.
DNA extraction and the use of RFLP markers were described in a previous paper (Crouzillat et al 1996). The Ecuadorian clones were compared to around 300 cocoa types from other origins. Data are presented here on the level of heterozygosity and on genetic diversity of all 416 genotypes.
Two agronomic traits were recorded for this study on the 29 “EB” (Escoba de Bruja) clones collected in 1994 and 1995 by INIAP and Nestlé within the scope of a cocoa “competition” to find witches’ broom resistant genotypes. The trunk diameter was assessed on each clone in 1997 (ten trees per clone) to estimate the vigour of the tree, The number of brooms per tree produced during the year 1997 was also counted.
For the evaluation of the genetic diversity, the RFLP profiles were scored by recording the presence or absence of each allele. Data were binary coded: 1 for the presence or o for the absence of each allele. Principal Component Analysis (PCA) was performed on the data sheet obtained using NCSS 2000 software. Each clone studied was assessed for heterozygosity level using 50 RFLP probes, by recording the different alleles at each locus. The average percentage of heterozygosity was calculated to give an indication of the overall genome heterozygosity. The allele frequencies were used to compute modified Rogers’ distance measures (Wright 1978) and to establish the dendrogram following the unweighed pair group procedure with arithmetic mean (UPGMA) (Sneath and Sokal 1973).
Detection of correlation between traits was performed by regression analysis, with a significant threshold of P = 0.05 using NCSS 2000 software.
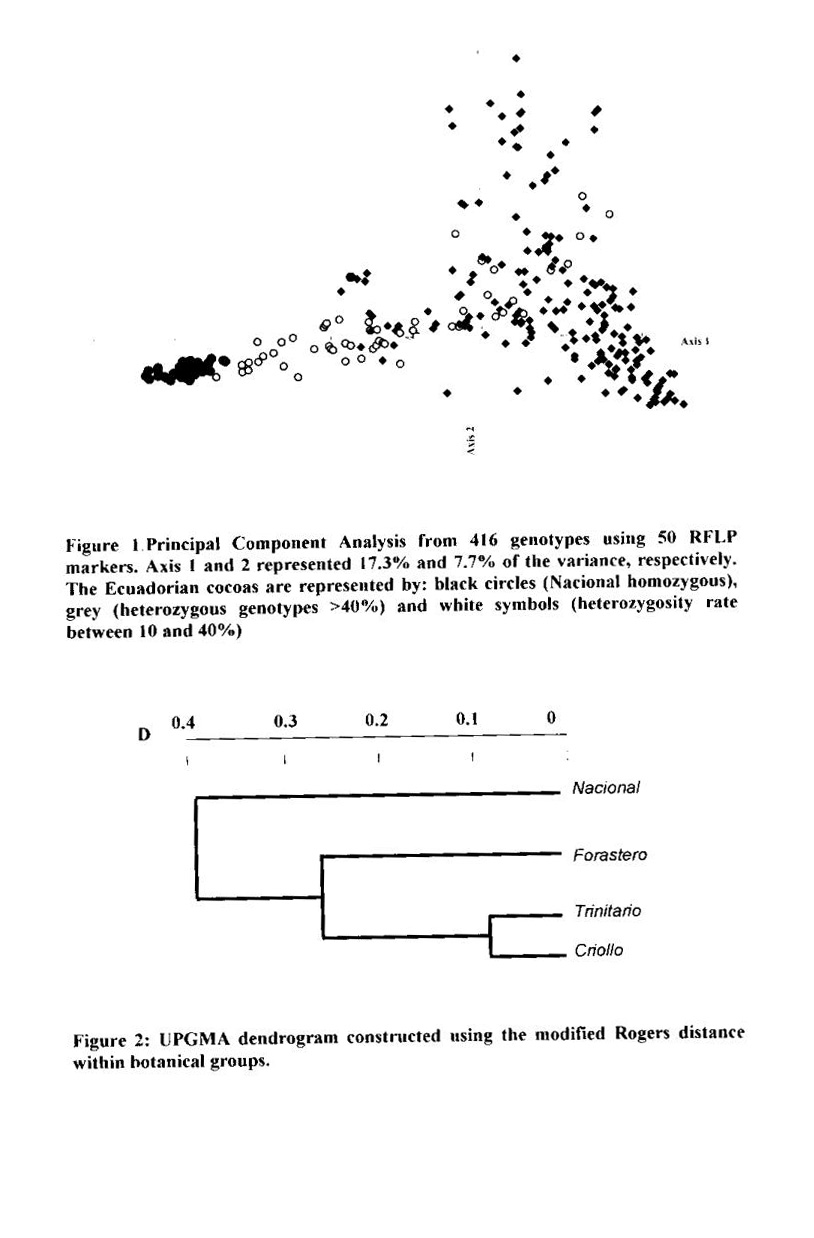
Genotypes from Ecuador. The RFLP analysis with the 50 RFLP probes indicated that only 45 probes were polymorphic with a total of 91 alleles. The average number of alleles per locus was 2.02. A global PCA study using the RFLP data was carried out with 416 different cocoa types from various origins. The resulting analysis (Figure 1) illustrates the genetic specificity of Ecuadorian cocoa types in comparison with the other genotypes. The estimation of RFLP heterozygosity level for each individual genotype (Annex 1) allows a better understanding of the genetic structure of cocoa in Ecuador and elsewhere. A cluster of Nacional genotypes with a low level of heterozygosity (5%) and a low genetic diversity was detected in the PCA study (Figure 1). These generally came from plantations which were more than 80 years old in Balao Chico (Guayas province) or Sebastian Arteaga (Manabi area). They could represent the germplasm originally associated with the reputation of the fine cocoa flavour called “Arriba”. Using cluster analysis, this genetic group was found to be different from classic botanical groups of Forastero and Criollo (Figure 2).
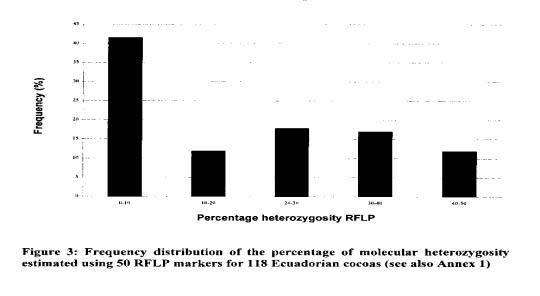
Other Ecuadorian cocoa genotypes were characterised by a higher level of heterozygosity (average 32%); values up to 44% were recorded for EET 46. EET 58, EET 62, EET 63 and EET 66. These genotypes could originate from crosses between Nacional types and types from Venezuela or Trinidad. The frequency distribution of Ecuadorian cocoas according to the heterozygosity level (Figure 3) illustrates the shift from the highly homozygous Nacional population to the presently predominating hybrid populations.
Cluster B contains exclusively Parinari genotypes from various collections, such as PA 13, PA 169 and PA 121. Pound collected these types for their apparent Witches’ Broom disease resistance from the region around Parinari (Peru) in 1937. These genotypes appear as moderately heterozygous (23.7% *1- 4.6%).
Cluster C mainly contains IMC and POUND genotypes. IMC (Iquitos Mixed Calabacillo) clones originated from pods collected by Pound in 1938 on two groups of trees which were free of witches broom disease. The POUND genotypes were also collected for witches broom resistance near the Nanay river. The heterozygosity level in this cluster is relatively high and variable (29.5% +1- 9.3%).
The location of the Peruvian genotypes is relatively scattered on the PCA plot (data not shown) and the Peruvian clones from the cluster A appear as the closest to the Ecuadorian types.
Arriba flavour evaluation. The first results of the sensory evaluation indicates that the cocoa trees with Arriba flavour (score _ 4) are neither distributed according to their geographic origin nor their genetic status. The Arriba clones were found from Manabi, El Oro, Los Rios and Guayas provinces independent of their heterozygosity level. These data suggest that Arriba flavour can be found either in “Nacional~ or hybrid types and that the Aruba flavour intensity is not related to the heterozygosity status of the genotypes analysed. In the same way, none of the four provinces, where the cocoa trees came from, gave significant differences in Arriba flavour intensity.

Crouzillat D., E. Lerceteau, V. Petiard, J. Morera, H. Rodriguez, D. Walker, W. Phillips, C. Ronning, R Schneil, J Osel and P, Ft 1996. Theobyoma cacao L.: a genetic linkage map and quantitative trait lad analysis. Theor AppI Genet 93: 205-214.
Enriquez GA, 1993. Characteristics of cocoa Nacional” of Ecuador. Pages 269-278 in Proceedings of the International Workshop on Conservation, Characterisation and Utilisation of Cocoa GenetIc Resourtes in the 21 century. Port of Spain, Trinidad. 13~17th September. The Cocoa Research Unit, The University of the West Indies.
Lachenaud P. and hi. Ducamp. 1996. Characteflsation and potential utilisation of wild cocoa from French Guiana. INGENIC Newsletter 2: 5-9.
Lerceteau E., J. Quiroz. J. Soria, S. Flipo, V. Petiard and a Crouzillat 1997. Genetic differentiation among Ecuadorian Theobroma cacao L. accessions using DNA and morphological analyses. Euphytica 95: 77-87.
Luz E.0. S.D. Silva, PS. Albuquerque, LR. Pinto, K.P. Gramacho. U.V~ Lopes, IL Pires, ML Brugnerotto and CA. Paim. 1999 Evaluation of cocoa progenies in Bahia, Brazil for resistance to Cnnipe!Iis pemniciosa. Pages 219-226 in Proceedings of the 12~ International Cocoa Research Conference, Salvador-Bahia, Brazil.
Petithuguenin P. and G. Roche. 1995. Ecuador: the cocoa sector, results and prospects Plantations Recherche, Développement 2: 22-26.
Pires J.L, W.R. Monteiro, ED. Luz, S.D. Silva, LR. Pinto, A. Figueira, KR Gramacho, U.V.
Lopes, P.S. Albuquerque, MM. Yamada, D.E Ahnert and Ml. Brugnerotto. 1999. Cocoa
breeding for witches broom resistance at CEPEC, Bahia, Brazil. Pages 91-102 in
Proceedings of the International Workshop on the Contribution of Disease Resistance to
Cocoa Variety Improvement. November 24-26, 1996, Sakador-Bahia, Brazil, INGENIC
1999, United Kingdom.
Sneath P.H.A and R.R. Sokal. 1973. Numerical taxonomy. Freeman, San Francisco, United States of America.
Van der Vossen, H, 1997. Strategies of variety improvement in cocoa with emphasis on durable disease resistance. Pages 23-32 in Proceedings of the International Workshop on the Contribution of Disease Resistance to Cocoa Variety Improvement. November 24-28, 1Q96, Salvador-Bahia, Brazil, INGENIC 1999, United Kingdom.
Wheeler BE. 1999. Host-pathogen interactions in witches broom disease of cocoa. Pages 83-90 in Proceedings of the International Workshop on the Contribution of Disease Resistance
to Cocoa Variety Improvement. November 24-26, 1996, Salvador-Bahia, Brazil, INGENIC
1999, United Kingdom.
Wright S. 1978. Evolution and the genetics of populations, vol 4~ variability within and among natural populations. University of Chicago Press, Chicago.
Zadoks J.C. 1997, Disease resistance testing in cocoa. Pages 17-22 in Proceedings of the
International Workshop on the Contribution of Disease Resistance to Cocoa Variety
Improvement. November 24-26, 1996, Salvador-Bahia, Brazil, INGENIC 1999, United
Kingdom.